
The design of the reactor used in hydrodealkylation can have a significant impact on the overall yield and selectivity of the conversion product. In this blog post, we use modeling and simulation to investigate the advantages of using a membrane reactor.
Understanding the Hydrodealkylation Process
From its use as an octane booster in fuels to its use as a solvent for paints and adhesives, toluene may be a substance with which you are already familiar. You may not realize, however, that this colorless, water-insoluble liquid is often referred to by another name — methylbenzene.
As the name suggests, the removal of a methyl group from toluene can be used to produce the chemical compound benzene. This process is known as demethylation.
Occurring at high temperature and pressure levels, this conversion takes place in the presence of a hydrogen gas within a reactor. During the chemical reaction, the available hydrogen replaces the removed hydrocarbon, resulting in a more simplified molecule — in this case, benzene. Note that at the same time, benzene can react reversibly to create the organic compound biphenyl. The term used to describe this hydrogen-intensive process is hydrodealkylation.
Now that we have a better feel for how this chemical reaction works, let’s take things one step further and analyze the use of a membrane reactor within the process.
Carrying out Hydrodealkylation with a Membrane Reactor
In hydrodealkylation, the belief is that a greater benzene yield can be generated by preserving higher concentrations of hydrogen. The addition of a membrane reactor helps to achieve these conditions as it supplies a continuous flow of hydrogen to the reactor across the porous membrane. The velocity of this hydrogen flow can be described by Darcy’s law. Aside from the hydrogen, no additional chemical species within the reactor pass through the membrane.
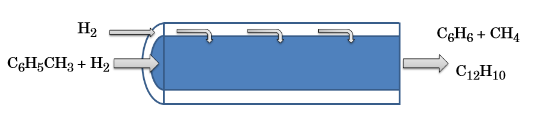
A schematic of the hydrogen flow through a porous membrane.
To model this process, we begin with the predefined Plug flow reactor type available in the Chemical Reaction Engineering Module. The reactor equations within this model can subsequently be modified to reflect the hydrogen moving across through the membrane.
Additionally, using the Thermodynamics feature, we can access property relations for the molar heat capacities and the molar enthalpies of the reacting species, solving for the energy balance. The Thermodynamics feature is a valuable resource as it enables you to connect with external databases of standard format for thermodynamic and physical property calculations. You can then incorporate this acquired information into your simulations.
Comparing Different Designs
To analyze the effectiveness of the membrane reactor in the hydrodealkylation process, we simulated two different reactor designs.
The first of these simulations involved a tubular reactor, which was supplied with equal molar flows of hydrogen and toluene. In this example, no hydrogen entered through the reactor circumference, with the reactant gas at the inlet maintained at 1200 K and 2 atmospheres.
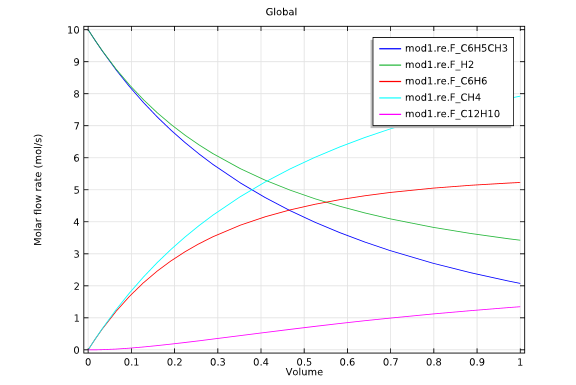
Model 1: Tubular reactor design.
The second study used a membrane reactor in the hydrodealkylation process. This model was fed with a constant supply of hydrogen through the membrane.
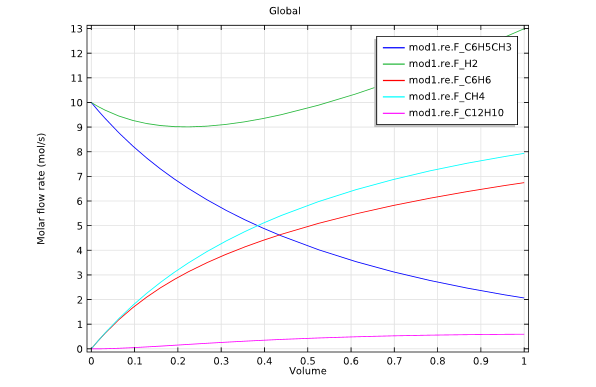
Model 2: Membrane reactor design.
The graphs above highlight the concentration distributions from each of the simulations. When comparing the results from each design, it is evident that the membrane reactor does, indeed, produce benzene with an increased selectivity.
Model Download
- Try it yourself: Download the Hydrodealkylation in a Membrane Reactor model from the Model Gallery





Comments (2)
Fernando Hortiz
May 19, 2016hi someone know how upload de coco program, I follow of tutotial and I don´t get upload de program. Help please.
Bridget Cunningham
May 19, 2016Hi Fernando,
Thank you for your comment.
You can download COCO from the following link: http://www.cocosimulator.org/.
Best,
Bridget