COMSOL Multiphysics® Bio-Cellular Tunneling Model
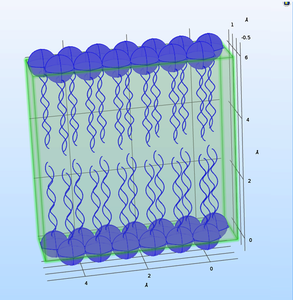
The emerging technologies related to AI and Quantum Computing are shifting the nano-bio-info paradigm, reconsidering the presently assumed bioenergetic processes of the living cells. Thus, a key process as ATPase can be considered through a model governed by quantum field theory (QFT) describing the how the activities in living cells are carried out though the protein conformational dynamics. As long as proteins are seeking for quantum coherence, and the donor-acceptor length fluctuations are generating phonon tunnelling effect (PCET) the collective effect matches the network-like behaviour at cell level and the signalling mechanism that occurs as a collective effect. Bose-Einstein condensation equations were used in Molecular Dynamics (MD) modeling research meant to describe the quantum superposition and quantum entanglement of proton/ phonon pumping mechanism. The MD model exported in MATLAB® was thereafter exported in COMSOL Multiphysics® through LiveLink™ for MATLAB® . A cell membrane model was designed in COMSOL Multiphysics® and its functional properties were described through CFD, Semiconductor, Heat Transfer and Structural Mechanics modules. The envisaged model has to describe ion channel gating pores, voltage-gated pores and the ATPase mechanism to create reliable nano-bio-info interfaces of the integrated modular-design concept for next generation of proactive biosensors.
References 1. A.A. Stuchebrukhov, Long-Distance Electron Tunneling in Proteins, Laser Phys. 20:125, (2010); doi.org/10.1134/S1054660X09170186 2. A. W. Chin, J. Prior, R. Rosenbach, et al., The role of non-equilibrium vibrational structures in electronic coherence and recoherence in pigment–protein complexes, Nature Physics volume 9, pages 113–118 (2013); doi.org/10.1038/nphys2515 3. E. Roberts, A. Magis, et al., Noise Contributions in an Inducible Genetic Switch: A Whole-Cell Simulation Study, PLoS Comput Biol 7(3): e1002010. (2011); doi.org/10.1371/journal.pcbi.1002010 4. E. Lacatus, Ion channel path of cellular transduction, Biochimica et Biophysica Acta (BBA) - Bioenergetics, Vol. 1837 (2014); doi.org/10.1016/j.bbabio.2014.05.266
Herunterladen
- lacatus_poster.pdf - 1.23MB
- lacatus_abstract.pdf - 0.6MB



