Finite Element Analysis of Chemical Reaction Kinetics and Transport Phenomena of a Solid State Combustion Synthesis
A 3D unsteady state chemical reaction system was modeled using COMSOL Multiphysics® simulation software. In this work, the Chemical Reaction Engineering Module was integrated to calculate physical, chemical and thermodynamic properties and transport phenomena.
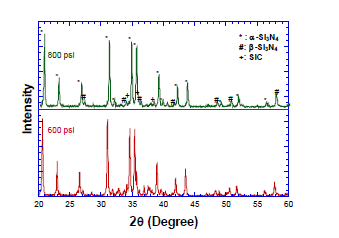
Advanced materials such as nitrides, oxides and carbides have been successfully synthesized in our lab by Self-propagating High-temperature Synthesis (SHS), which is a highly exothermic reaction process. Its theoretical adiabatic temperature can reach 3,000 K. Because of the process’s high reaction temperature, fast heating/cooling and reaction rate the development of a mathematical simulation is crucial for a safe large scale manufacture. In this work, a time dependent three-dimensional finite element analysis model was developed to study the synthesis of silicon nitride via 3 Si + 2 NaN3 + 0.2 Si3N4→ 1.2 Si3N4 + 2 Na + N2. This is an unsteady state solid state combustion. An eight step reaction mechanism consisting of silicon nitridation, sodium azide decomposition, sodium vaporization and silicon nitride decomposition at high temperatures was used to calculate local reaction rates and composition.
In our model instaneous local composition, reaction kinetic, thermodynamic and transport properties (reaction rate constant, heat capacity, density, viscosity, thermal conductivity, and temperature) changed continuously and influenced each other. These properties were calculated by reported data as well as the reported activation energies for each of the eight steps.
Temperature history, reaction rates, reaction conversion, combustion front movement velocity, and the impact of initial silicon nitride diluent on the SHS process were studied using the COMSOL built-in momentum (Navier-Stokes equation) and heat transfer (conduction, convection and radiation) equations.
Figure 3 shows some examples of our calculated results. Figure 3(a) is the temperature distribution along the centerline of the reaction pellet at different times. Figure 3(b) is the chemical compositions at the center point of the reaction pellet.
The dynamic velocity of reaction front movement, the temperature and composition histories, and the impacts of silicon nitride initially added to the reaction system will be presented.
Herunterladen
- lin_paper.pdf - 1.04MB
- lin_chem2_presentation.pdf - 1.94MB
