Simulating Survival and Insulin Secretion in Pancreatic Islet Tissue Constructs
Type I diabetes results from the autoimmune destruction of pancreatic islets and is a growing and cost intensive chronic health problem throughout the world. Monitoring blood sugar levels and recurrent intervention with exogenous insulin allows many patients to lead relatively normal lives. Despite this, diabetes still causes numerous short-term complications such as hyperglycemic episodes, and long-term complications such as cardiovascular disease and diabetic nephropathy.
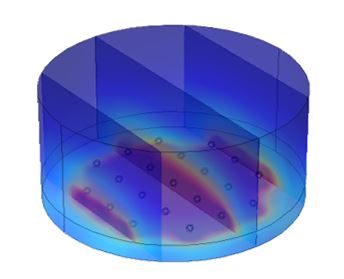
Transplantation of pancreatic islets which secrete insulin can restore endocrine control of blood sugar levels and provides patients with improved glycemic control to avoid the debilitating side effects of diabetes. Unfortunately, the translation of many islet therapies from rodent models to humans has largely been met with failure since scaling up many techniques has not been suitably considered from the theoretical standpoint. Designing better techniques and tissue constructs for human islet transplantation requires carefully considering the local islet environment. A detailed and accurate mathematical model of mass transfer can be used to reconstruct islet transplantation environments in silico to predict islet transplantation success in vivo. The islets themselves can be modeled using equations with COMSOL’s chemical reaction module to describe the glucose-dependent production of insulin and the consumption of oxygen and glucose. Tracking the diffusion of oxygen, glucose, and insulin using COMSOL’s transport of diluted species module throughout tissue construct settings can then provide an effective way of simulating whether an islet tissue construct will be successful.
While previous papers have simulated single islet dynamics, few studies have simulated the effectiveness of multiple islets in large tissue constructs. To learn more about the requirements of pancreatic islets in tissue constructs designed for transplantation, three variables will be investigated in this study: hydrogel diffusion coefficient, islet density, and islet vascularization.
Common islet tissue constructs will seed multiple islets into hydrogels before implantation. The hydrogel diffusion coefficients for oxygen, glucose, and insulin can drastically affect islets seeded into the hydrogel. For example, a low diffusion coefficient of oxygen may lead to islet death and transplant failure while a low diffusion coefficient of insulin may prevent the islets from secreting insulin into the bloodstream of the patient quickly enough. Islet survival and functionality sensitivity will be investigated by varying diffusion coefficients in tissue construct settings to determine optimal values. In larger tissue constructs with multiple islets, the density of islets also plays a critical role. A higher density allows for more islets in a single tissue construct but may lead to hypoxic areas. Finally, native islets are highly vascularized tissues and the presence of vascular networks highly affects the transport dynamics of oxygen, glucose, and insulin. Blood flow and vascular connectivity will be simulated with COMSOL’s fluid flow modules to determine the effect of islet vascularization on transplantation success.
Investigating the requirements of pancreatic islets with respect to these three variables can help to elucidate the behaviors of islet transplantation tissue constructs in silico. Researchers can utilize the results of these simulations to design more effective islet transplantation technologies capable of ensuring islet survival and adequate functionality through insulin secretion.
Herunterladen
- han_poster.pdf - 0.74MB
- han_abstract.pdf - 0.02MB



