Die Application Gallery bietet COMSOL Multiphysics® Tutorial- und Demo-App-Dateien, die für die Bereiche Elektromagnetik, Strukturmechanik, Akustik, Strömung, Wärmetransport und Chemie relevant sind. Sie können diese Beispiele als Ausgangspunkt für Ihre eigene Simulationsarbeit verwenden, indem Sie das Tutorial-Modell oder die Demo-App-Datei und die dazugehörigen Anleitungen herunterladen.
Suchen Sie über die Schnellsuche nach Tutorials und Apps, die für Ihr Fachgebiet relevant sind. Beachten Sie, dass viele der hier vorgestellten Beispiele auch über die Application Libraries zugänglich sind, die in die COMSOL Multiphysics® Software integriert und über das Menü File verfügbar sind.
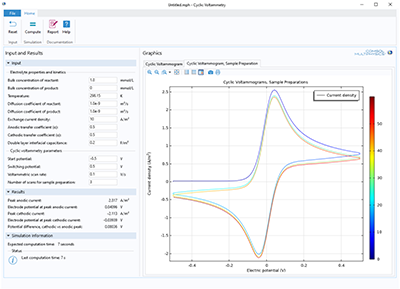
Zweck dieser App ist es, die Anwendung der Cyclovoltammetrie zu demonstrieren und zu simulieren. Sie können die Massenkonzentration beider Spezies, die Transporteigenschaften, die kinetischen Parameter sowie das Zyklusspannungsfenster und die Scanrate variieren. Die Cyclovoltammetrie ... Mehr lesen
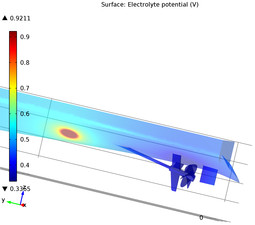
Impressed current cathodic protection is a commonly employed strategy to mitigate the ship hull corrosion where an external current is applied to the hull surface, polarizing it to a lower potential. In this model, the effect of propeller coating on the current demand is demonstrated. Mehr lesen
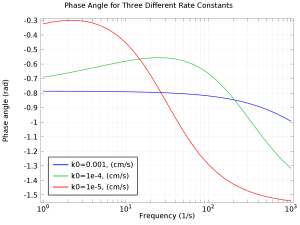
Der Zweck dieser App ist es, EIS-, Nyquist- und Bode-Plots zu verstehen. Mit der App können Sie die Volumenkonzentration, den Diffusionskoeffizienten, die Austauschstromdichte, die Doppelschichtkapazität sowie die maximale und minimale Frequenz variieren. Die elektrochemische ... Mehr lesen
This tutorial models the cathodic protection of an oil rig structure during a time period of 15 years. As a result of the consumption of the sacrificial anodes, the protective capabilities system are reduced over time. The anode shape change in the model is defined by using the Level ... Mehr lesen
The Cathodic Protection Designer application is an example of how an application can be used to simplify the simulation process by featuring a way to import a generic CAD file with certain requirements. Using this app, the user can select each part of the geometry and set boundary ... Mehr lesen
Pitting corrosion is a type of localized corrosion by which local cavities, pits, are formed on an initially smooth metal surface. A pit may be initialized by surface defects, such as an inhomogeneities in composition or shape, or mechanical abuse resulting in a small scratch or dent. ... Mehr lesen
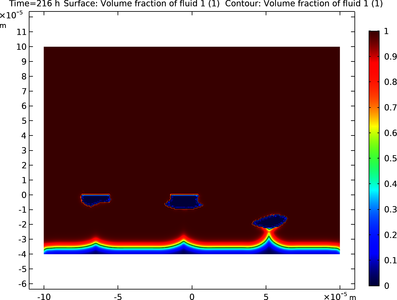
This example models galvanic corrosion between two different phases in a magnesium alloy for a representative cross-sectional microstructure configuration. The Level Set interface is used here to model dissolution of a constituent phase leading to topological changes. The electrode ... Mehr lesen
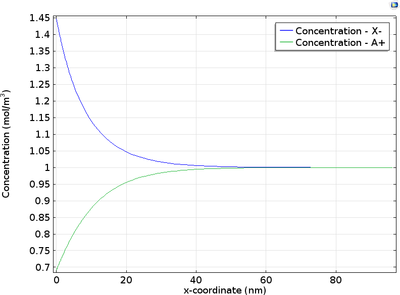
An der Elektroden-Elektrolyt-Grenzfläche befindet sich eine dünne Schicht von Raumladungen in einer diffusen Doppelschicht. Dies kann bei der Modellierung von Geräten wie elektrochemischen Kondensatoren und Nanoelektroden von Interesse sein. Dieses Tutorial-Beispiel zeigt, wie man die ... Mehr lesen
Biodegradable metallic biomaterials such as Magnesium (Mg) are gathering attention for biomedical applications due to their favorable properties. The present model simulates dissolution of a Mg stent in a blood vessel. Due to the circumferential symmetry, only one-twelfth of the actual ... Mehr lesen
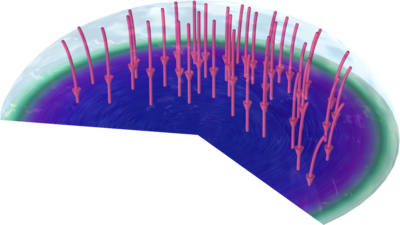
An Evans droplet experiment is a century-old corrosion experiment for demonstrating oxygen transport-limited corrosion. A droplet of water is placed on a metal surface, and over time the surface features differences in the radial direction of the surface in terms of amount of corroded ... Mehr lesen