A Multiscale Model of the Bipolar Electrode - SDS Adsorption on Stainless Steel
In solution, sodium dodecyl sulfate (SDS) (structure shown in Fig. 1) can form a boundary film on metal surfaces. Previous studies have extensively investigated the characteristics of such surfactant films experimentally [1][2][3]. A dependency of SDS surface concentration and film structure on the metal's electric potential has been demonstrated, in aqueous as well as in non-aqueous solutions. What is more, such alterable film structure allows active friction control. In the particular case of aqueous and non-aqueous SDS solution on stainless steel, a phase transition in film structure from flat lying dodecyl sulfate monomers to hemicylindrical aggregates such as depicted in Fig. 2 (a) and (b) is observed when gradually increasing SDS surface concentration by tuning surface potential or bulk concentration. This phase change happens within a critical potential interval, which depends on bulk concentrations, solvent characteristics and electrode material.
Employing a bipolar electrode (BPE) as shown in Fig. 3 (a), the film's phase transition can be resolved spatially due to potential gradient across the electrode surface [3]. While many works focus on the experimental quantification of such surface films and their phase transitions, less has been done to explain and validate the results from a theoretical point of view.
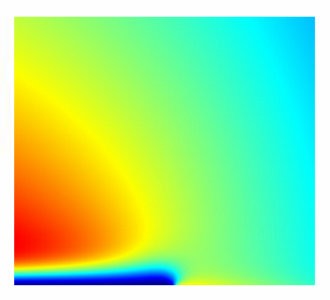
In our work, a continuous multiscale model of the bipolar electrode is developed, particularly incorporating faradaic reactions and microscopic double layer structure present at the idealized aqueous solution / BPE interface. The approach is based on solving a classical Poisson-Nernst-Planck equation system with help of the "Tertiary Current Distribution", "Electrostatics" and "Transport of Diluted Species" as well as "Partial Differential Equations" functionality of COMSOL Multiphysics® software. The novelty of our work lies in spanning several scales and treating model features explicitly over all scales, such as concentration distributions both across the whole electrochemical cell at macroscopic scales of millimeters and across the electrical double layer at surface at microscopic scales of several nanometers. Thereby, the potential's deviation from linear behavior typical for BPE as shown in Fig. 3 (b) is reproduced. Fig 3 (c) shows the potential difference between BPE and adjacent electrolyte as measured by a voltmeter such as sketched in Fig. 3(a). More important, accurate concentration profiles at surface are found.
This contribution should be understood as an essential first step in order to realize a thorough multiscale model of the film formation at steel surface. Based upon the continuous macroscopic features predicted, a discrete nanoscale model should be used to find the surface film’s molecular structure in subsequent studies.
Herunterladen
- hörmann_poster.pdf - 1.15MB
- hörmann_abstract.pdf - 0.08MB



