Membrane Electrode Assembly for CO2 Electroreduction: Influence of Flow Channel Design
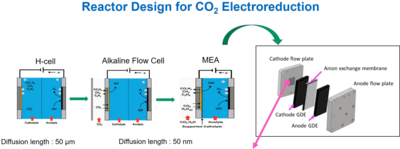
Electrochemical reduction of CO2 to value added chemicals is a promising strategy to mitigate its emission from atmosphere and close the carbon cycle. Although the knowledge on designing electrocatalysts for CO2 electroreduction exists, challenges are associated with developing CO2 electrolyzers at high current densities (> 200 mA/cm2) which are relevant for industrial operation. While several reactor configurations exist, a membrane electrode assembly (MEA) comprising of an ion exchange membrane sandwiched between two gas diffusion electrodes seems to be a promising design for scale up. In this reactor configuration, a uniform distribution of CO2 and electrolyte to the catalyst surface is a crucial factor to enhance its performance and avoid hot spots from forming which reduces the overall performance. To accomplish this, the flow channel design on the bipolar plate of the MEA has to be optimized and much better understood. Such understanding leads to experimental improvements by ensuring uniform reactant distribution, while also facilitating the transport of electrons and products from the reaction sites. In this study, the cathode of the MEA with a serpentine flow channel was modelled using COMSOL Multiphysics® and simulation was performed to obtain the concentration of CO2 on the cathode surface. The 3D model comprised of a serpentine flow channel in contact with a gas diffusion layer (GDL) and a 2 electron electrochemical reduction of CO2 to CO was studied. The multiphysics feature ‘Reacting flow in porous media’ that couples the Brinkmann equations with the Transport of concentrated species (TCP) interface was incorporated in this study. A no slip boundary condition was used in the channels and a slip condition in the GDL. The properties of the GDL such as the porosity and permeability where obtained by using Sigracet 39BC carbon GDL from Fuel Cell Store as the reference. In the TCP interface, the mixed average diffusion model which combines Stefan Maxwell diffusivity and Knudsen diffusivity was utilized. The binary diffusion coefficients of reacting species were calculated using Fuller Correlation and the electrochemical reduction reaction was studied on the cathode side of the GDL. The 'Electrode surface coupling' feature in the TCP interface was used to model the electrochemical reaction and a current density of 200 mA/cm2 was applied on the Cathode. Simulation was performed at steady state conditions using a free tetrahedral mesh for the channels and a swept mesh for the GDL. The simulation results showed that the concentration of CO2 decreases from the inlet of the channel to the outlet creating hotspots on the cathode surface. The results obtained show the influence of flow channel design on the performance of an MEA which aids in formulating design rules for industrially relevant CO2 electrolyzers.