Modeling the Chemical Decomposition of Sodium Carbonate Peroxyhydrate
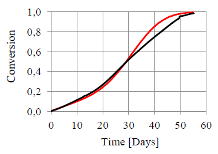
The challenge with the use of Sodium Carbonate Peroxyhydrate (Na2CO3*1.5H2O2) as a bleach source in dry detergent formulations is its lower stability in comparison with other materials, and the risk of quality losses of the product over the shelf life. The issue can be solved with the understanding and modeling of the decomposition mechanism of the powder. It is well known that the decomposition mechanism is quite complicated and involves a number of species, reactions and physical transformations; it is faster at high temperature and catalyzed by water and some metal ions. Furthermore, previous studies have shown the existence of different reaction mechanisms in different experimental conditions. The case study presented in this work deals with the chemical decomposition of pure Sodium Carbonate Peroxyhydrate (SPC), and is a part of a wider analysis aiming at the simulation of the decomposition of a dry laundry detergent inside a pack. The full model, still under development, involves the moisture diffusion through the pack (see separate citation), moisture adsorption in the product and the chemical decomposition. The innovative application of COMSOL Multiphysics in this context is to use of the chemical reaction engineering module to develop a zero order model of the decomposition of a bed of particles. Indeed, we developed a time dependent study of the constant volume batch reactor involving five different species: Sodium Carbonate Peroxyhydrate, Anhydrous and Monohydrate Sodium Carbonate, Water and Hydrogen peroxide; and involving three solid state and two homogeneous reactions. Modeling this kind of systems is usually complicated because of the changes at particle scale and the phenomena occurring in the solid state. Nevertheless, we approached the description of such complexity trying to avoid the development of a complicated 3D model. In order to keep the computational simplicity, and at the same time have a good description of the entire process, we introduced in the module the appropriate solid state reaction kinetic models. These describe the variation of the reaction rate with conversion and easily allow the introduction of complex phenomena such as reagent and product diffusion, particle shrinkage, crystal granulation and growth. The parameters used were partly estimated, partly taken from the literature or extracted from the kinetic analysis of the system carried out in our laboratories. By means of this model we are able to reproduce the decomposition of Sodium Carbonate Peroxyhydrated over the shelf life. Furthermore, we are able to describe the decomposition in different conditions and simulate the switch from diffusion controlled to autocatalytic decomposition, with small changes in initial and boundary conditions. Indeed, despite of the computational simplicity of this study, the results are in good agreement with the experimental data.
Herunterladen
- brundu_paper.pdf - 0.45MB
- brundu_abstract.pdf - 0.02MB



