Bubble Detachment from the Surface of a (Photo)Electrode
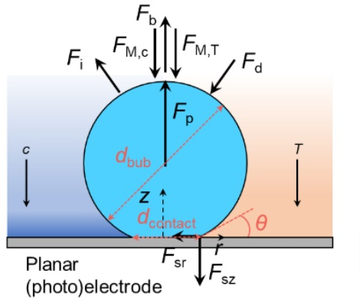
Many (photo)electrolytic devices involve the generation of bubbles (e.g., hydrogen, oxygen). In these devices, bubble coverage on the surface of the electrode not only decreases the active area, but also induces optical loss.[1,2] Efforts in understanding the bubble detachment process and the influencing parameters are therefore important in minimizing the losses. For example, a super-saturated boundary layer is built up near the electrodes during the (photo)electrochemical process.[3] The properties of this special boundary layer (e.g., thickness, super-saturation level) have been well studied, the impact of the super-saturated dissolved gas on bubble detachment, however, is often overlooked. Here, we numerically investigate the detachment of an oxygen bubble from a planar (photo)electrode in the presence of a pre-defined super-saturated boundary layer of dissolved oxygen. The morphological change of the liquid/gas interface was simulated using the Level-set method, and the effects of different super-saturation levels, the thickness of the super-saturated boundary layer, and the contact angle on the bubble detachment were investigated. Our results show that bubble fails to detach from the electrode when the super-saturated boundary layer of dissolved oxygen is ignored (Fig. 1a). The same bubble successfully detaches when the impact of the super-saturated boundary layer is included (Fig. 1b), indicating the importance of the super-saturated boundary layer. This difference arises since the concentration gradient induces a surface tension gradient, which means that an additional force (i.e., the Marangoni force) needs to be considered. This force leads to dramatic morphological changes during bubble detachment, see Fig. 1b. A more flattened bubble shape (see the difference in Fig. 1a and 1b) results in a decrease of the surface tension force, which leads to facilitating bubble detachment. Overall, the findings from this work not only elucidate the mechanisms of how the super-saturated boundary layer affects (photo)electrolytic bubble detachment, but also provide practical suggestions for cell or electrolyte engineering.
Herunterladen
- Liang_6301_presentation.pdf - 2.94MB
- Liang_6301_poster.pdf - 1.82MB
