Electrical Modeling of Molten Salt Electro-Refining Processes
A common pyrometallurgical route for the recovery of numerous metals and rare earths is high-temperature molten salt electrolysis. This process involves an electrolyte made of a molten salt in which the metal to be recovered, most commonly present in its oxide form, is dissolved. When a current is applied between the cathode and anode, the metal is deposited as a solid or a liquid at the cathode, while some gas (generally a carbon oxide, such as CO or CO2) evolves at the carbon-based anode, as depicted in the figure below.
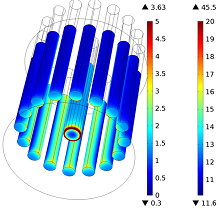
Numerical simulation can be advantageously employed to predict the main cell features (e.g., the reaction rate distribution on the electrodes, the cell voltage, or the electrolyte temperature) in order to optimize the design and operational conditions of the process. Even though high-temperature electrolysis involves many physical phenomena that are complex and strongly coupled to one another, the reaction rate distribution on the electrodes can be approximated with a rather simple electrostatic approach that involves the calculation of the current densities associated with the reactions.
In this presentation, we describe a computational approach for predicting current distributions in a molten salt electro-refiner. The three main types of current distribution (primary, secondary, and tertiary) are addressed, with a particular emphasis on primary and secondary. A simple approach to implement current-predicting models for electrolysis cells in COMSOL Multiphysics® software is presented. The simulated current at the electrodes’ surface (the reaction rate) are analyzed and the main differences between the primary and the secondary description are explained.
Herunterladen
- oury_presentation.pdf - 1.57MB
- oury_paper.pdf - 0.77MB
- oury_abstract.pdf - 0.04MB



