Lithium-Ionen-Akkus
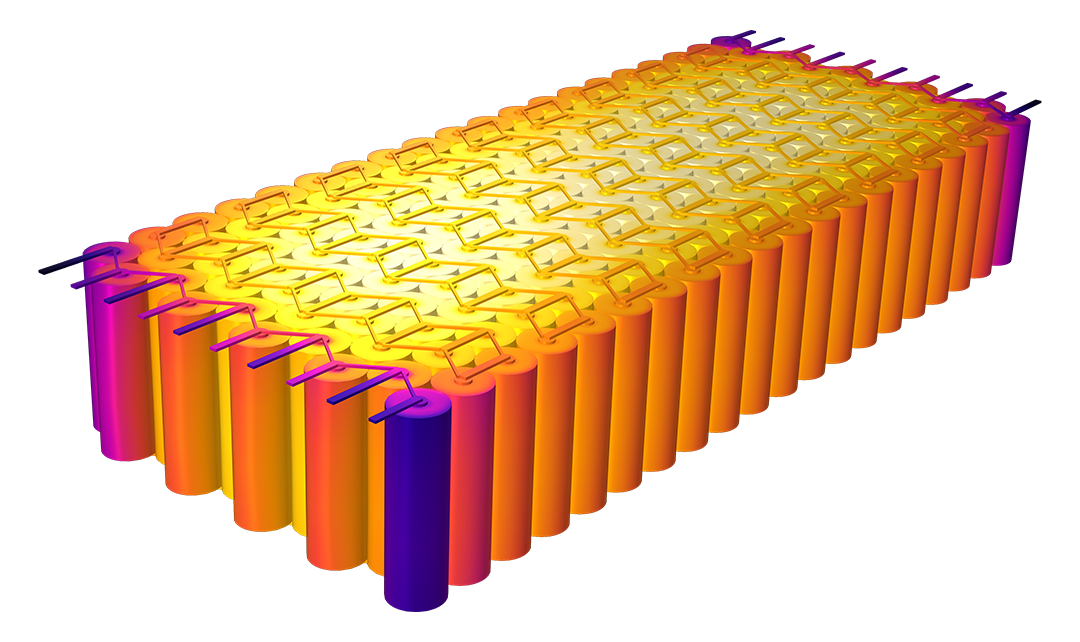
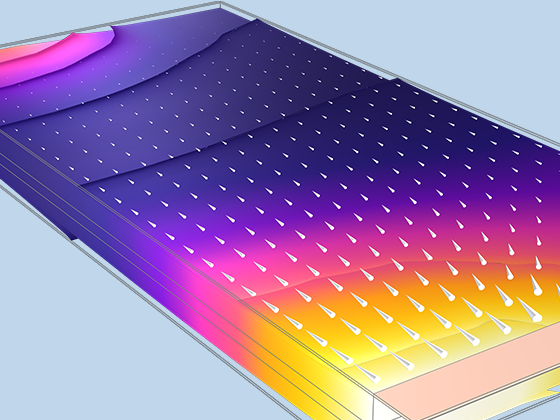
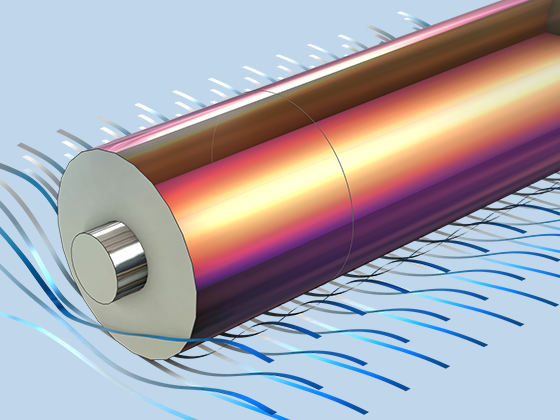
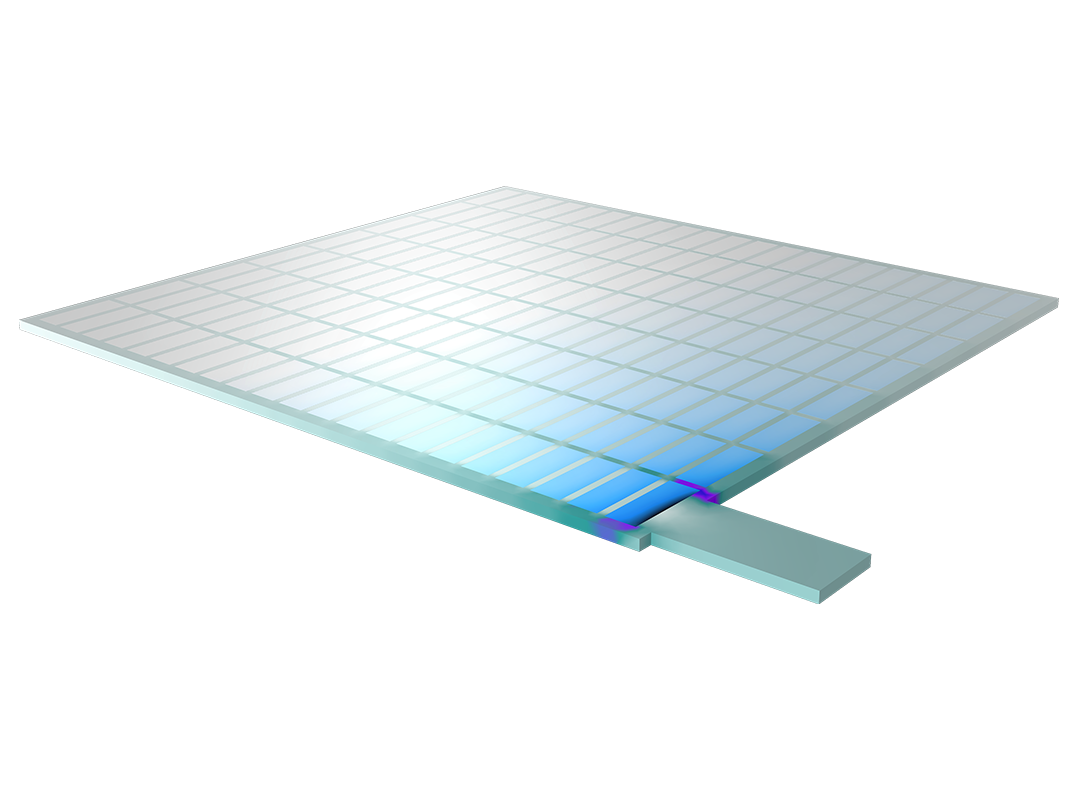
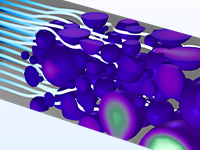
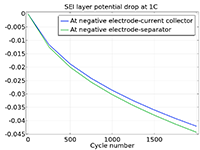
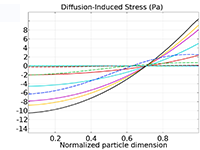
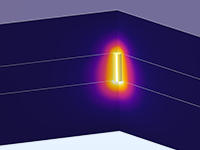
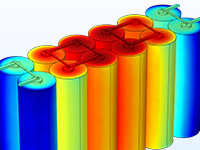
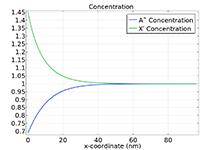
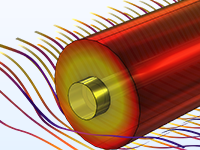
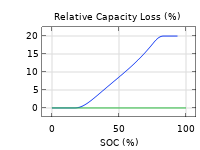
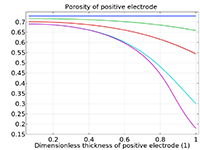
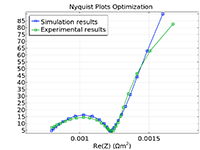
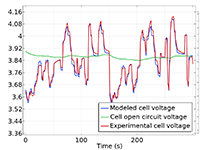
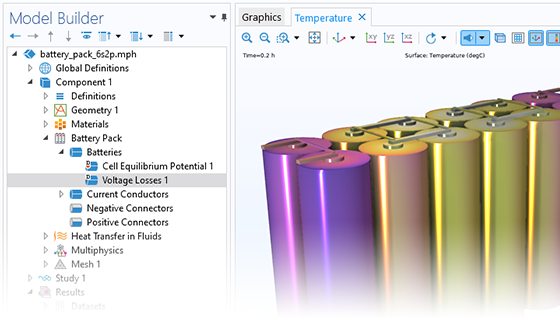
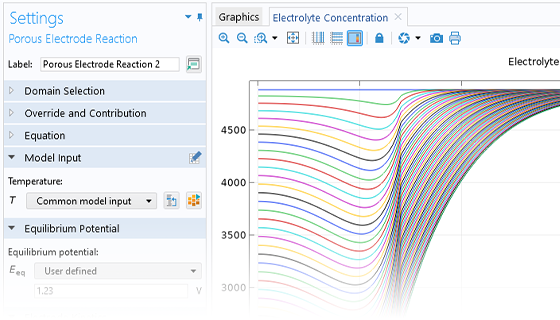
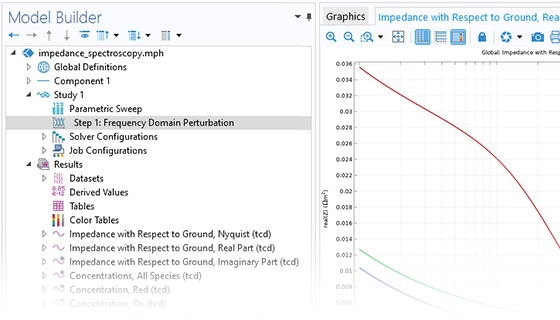
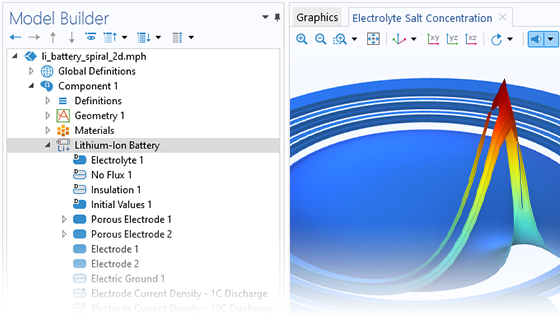
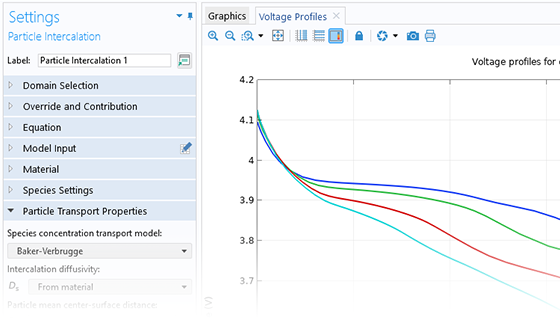
Das Battery Design Module bietet modernste Modelle für Lithium-Ionen-Akkus. Sie finden verschiedene Mechanismen für die Alterung und hochrealistische Modelle, wie das Newman-Modell, das in 1D, 2D und 3D verfügbar ist. Sie können nicht nur die elektrochemischen Reaktionen allein modellieren, sondern diese auch mit dem Wärmetransport kombinieren und die strukturellen Spannungen und Dehnungen berücksichtigen, die durch die Ausdehnung und Kontraktion bei der Lithiumeinlagerung entstehen. Das Modul bietet auch Funktionen zur Erstellung heterogener Modelle, die die tatsächlichen Formen des Porenelektrolyts und der Elektrodenpartikel beschreiben. Die Untersuchung der Mikrostruktur eines Akkus trägt zu einem tieferen Verständnis der Akkuleistung bei.